Anisocytosis with hypochromia and microcytes (IDA)
Iron-deficiency anemia (IDA) is the most commonly encountered anemia and may be due to [1] impaired iron intake, [2] pregnancy, [3] intravascular hemolysis, [4] hemorrhage, or [5] lactation. IDA most often affects women in their reproductive years and growing children. Each mL of packed RBC’s contain about 1.0 mg of iron. The average adult contains about 3.5 to 5.0 grams of iron. They will ingest about 15 to 20 mg of iron daily, excreting most of it. The body normally absorbs about 1 mg of iron (which is equal to the daily loss).
The iron taken into the body is in the ferric state (Fe+3) in the stomach, it is changed to the ferrous state (Fe+2). Ferrous iron is absorbed by the small intestine and in the intestinal capillary system, iron is bound to transferrin to form a protein-iron complex. This complex is carried to the bone marrow (and other cells requiring iron) and will bind to the cell. The complex is absorbed into the cell, the iron released, and the transferrin moves back into the blood stream. Inside the cell, the iron is bound to the protein apoferritin to form ferritin. The ferritin combines to form aggregates which forms brown pigment granules called hemosiderin. IDA is a hypoproliferative, microcytic, hypochromic anemia due to ineffective RBC and/or hemoglobin production.
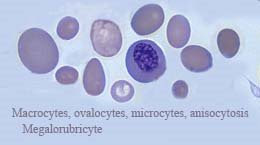
If IDA is observed in a healthy appearing adult male, the physician should look for a gastrointestinal lesion that may be losing blood. The menstruating woman will lose between 50 and 70 mLs of blood monthly and when the iron is not being replace anemia will result. It is estimated that up to 20% of the women in the US have IDA. IDA produces a hypochromic, microcytic anemia. On the peripheral blood smear, the erythrocytes are hypochromic and microcytic. If IDA is severe, poikilo-cytosis and anisocytosis may be obvious. In the bone marrow, the rubriblasts will be poorly hemoglobinized and demonstrate ragged appearing cytoplasm. The serum iron will be decreases and the total iron binding capacity (TIBC) will be increased. The ferritin level will be less than 20 ng/mL.
IRON TRIVIA
If dietary iron is from meat sources, it is heme bound. Vitamin C is not required for absorption. If this iron is from eggs and vegetables, it is in the ferritin and hemochrome bound form and requires vitamin C for optimum absorption. In the stomach, the gastric fluid and pepsin releases iron which passes into the gastrointestinal tract. Most iron absorption occurs in the duodenum. Some iron absorption will occur in the jejunum and ileum.
In developed countries, adequate ion intake is not a problem. The high risk groups who are most likely to develop IDA are [1] infants, [2] rapidly growing adolescents, [3] pregnant women, and [4] women during their child bearing years (losing from 10 to 45 mg/month). The pregnant female need about 3.4 mg of iron daily (a total of 1000 mg to carry the fetus to term). About 400 mg are needed for the fetal RBC mass. At parturition, approximately 300 mg will be lost and up to 170 mg will be contained in the placenta and umbilical cord.
It has been estimated that a healthy adult male would require about eight years to develop IDA if no more iron were absorbed in his diet. Malabsorption is uncommon unless there is a primary problem as [1] sprue, [2] gastrectomy, or [3] atrophic gastritis. Other causes are [1] regular blood donations and [2] paroxysmal nocturnal hemoglobinuria.
CLINICAL SYMPTOMS
Early stages (stage 1) are generally asymptomatic. As IDA develops into stage two, the depletion of the body’s iron stores occurs, with the patient experiencing hypoxia, characterized by lethargy and asthenia. As stage two progresses, iron deficiency is demonstrated by a decrease in erythropoiesis as iron is no longer being inserted into the hemoglobin molecule. Lab testing will show decreases in serum iron, increased total iron binding capacity (TIBC), and low transferrin saturation.
As the IDA progresses into the stage three level, the mitotic activity of the RBC increases resulting in small erythrocytes (microcytes) and hypochromia. When the hypochromic microcyte is observed in the blood film, there is also anisocytosis and poikilocytosis and IDA if fully expressed. Symptoms that begin to appear in stage two and are fully expressed in stage three are [1] ankle edema, [2] exertional dyspnea, [3] headaches, [4] glossitis, [5] koilonychia, [6] pallor, [7] pica, and [8] tachycardia. In the woman of child bearing age, [1] menorrhagia, [2] irregular cycles, and/or [3] amenorrhea may occur.
CLINICAL LABORATORY FINDINGS
RBC count: Usually normal at the beginning. The count will usually remain within normal limits unless the iron stores are severely depleted.
Hemoglobin: Will undergo greater degrees of reduction. Individual are to be considered anemic if hemoglobin values fall as indicated in g/dL:
- [1] Children (from 6 months to 5 years) less than 11
- [2] Children (from 6 years to 14 years) less than 12
- [3] Adult men. . . . . . . . . . . . . . . . . . . . . . less than 13
- [4] Adult women. . . . . . . . . . . . . . . . . . . less than 12
- [5] Pregnant women . . . . . . . . . . . . . . . . less than 11
RBC Indices: Anemia is suspected when the values fall as indicated:
- [1] MCV . . . . . 75 to 80 fL
- [2] MCH . . . . . 25 to 27 pG
- [3] MCHC. . . . less than 32 percent
- [4] If the MCV is less than 75 fl.
- [5] If the MCH is less than 25 pG
Retic Count: This test parameter may be normal or decreased in early IDA. As the IDA progresses, the retic count decreases.
The Reticulocyte Production Index is a better indicator of bone marrow responses in anemia.
Fragility: This test will usually be normal. If codocytes (target cells) are present, then one may see a decreased value. The value of this test is in detecting hereditary spherocytosis.
WBC: The count is usually normal as is the differential.
Platelet count: This testing parameter is usually normal.
Serum Iron: decreases in stages as IDA develops.
Normal values (μg/dL) as follows:
- Newborn . . . . . . . 100 to 250
- Infant . . . . . . . . . 40 to 100
- Child . . . . . . . . . 50 to 120
- Adult male. . . . . . 65 to 170
- Adult female . . . . 50 to 170
Serum Ferritin: Is an indicator of how much iron is being stored and it will progressively decrease as IDA develops. It is the major iron storage compound and is found in all body cells. It is a protein that is complexed with iron. If iron is absent from this protein, it is then know as apoferritin. This is an important test in differentiating IDA from other types of microcytic normocytic anemias as it will be increased in thalassemia and sideroblastic type anemias. Normal values for men are 15 to 200 μg/L and women are 12 to 150 μg/L.
Free erythrocyte protoporphyrin (FEP): This test provides the same information as the serum ferritin. Protoporphyrin is the compound to which ferrous iron is added to form the heme molecule. In normal hemoglobin production, a little more protoporphyrin is produced than is required. When there is an iron deficiency, protoporphyrin will increase in the RBC. The normal reference is less than 50 μg/dL of RBC.
Generalized test findings as IDA develops.
[1] In the initial stages when patient is asymptomatic:
- A. Serum ferritin will be decreased
- B. Bone marrow iron will be decreased
[2] In the second stage, when erythropoiesis is occurring without
iron to insert in the heme portion of the hemoglobin molecule:
- A. Serum Ferritin continues to be decreased.
- B. Bone marrow iron continues to be decreased.
- C. Serum iron is now decreased.
- D. TIBC is increased.
[3] In the final stage with fully developed IDA:
- A. Serum Ferritin, bone marrow iron, and serum iron are decreased.
- B. TIBC is increased.
- C. Hemoglobin and hematocrit are decreased.
- D. MCV is decreased.
- E. RDW is increased.
Bone marrow findings in IDA. NOTE: Bone marrow studies are usually not required to diagnosis IDA.
[1] Mild to moderate erythroid hyperplasia.
[2] Increase in number of cells in the erythroid line.
[3] Look for smaller rubriblasts (normoblasts) with [a] pyknotic nucleus, [b] thin rim of irregular basophilic cytoplasm, [c] the absence of or decreased hemoglobiniz nuclear an[a dding, developed IDA, stainable iron granules are absent.